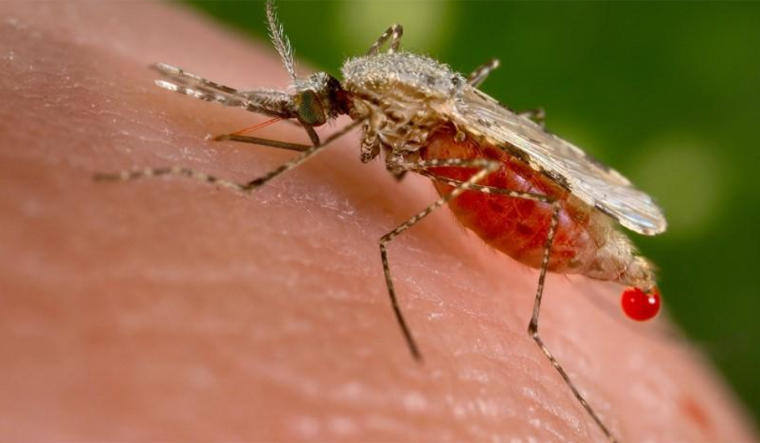
WASHINGTON: A fungus genetically modified to produce spider toxin can rapidly kill over 99 per cent of the mosquitoes that spread malaria, a study has found.
According to the World Health Organization (WHO), malaria affects hundreds of millions of people around the world, killing more than 400,000 annually.
Decades of insecticide use has failed to control mosquitoes that carry the malaria parasite and has led to insecticide-resistance among many mosquito strains.
In response, scientists began genetically modifying mosquitoes and other organisms that can help eradicate mosquitoes.
Until now, none of these transgenic approaches made it beyond laboratory testing.
Scientists from the University of Maryland (UMD) in the US carried the first trial outside the laboratory of a transgenic approach to combating malaria.
The study, published in the journal Science, showed that a naturally occurring fungus engineered to deliver a toxin to mosquitoes safely reduced mosquito populations by more than 99 per cent in a screen-enclosed, simulated village setting in Burkina Faso, West Africa.
“No transgenic malaria control has come this far down the road towards actual field testing,” said Brian Lovett, a graduate student at UMD.
“This paper marks a big step and sets a precedent for this and other transgenic methods to move forward,” Lovett said.
“We demonstrated that the efficacy of the transgenic fungi is so much better than the wild type that it justifies continued development,” said Raymond St Leger, a professor at UMD.
The fungus is a naturally occurring pathogen that infects insects in the wild and kills them slowly. It has been used to control various pests for centuries.
The scientists used a strain of the fungus that is specific to mosquitoes and engineered it to produce a toxin that kills mosquitoes more rapidly than they can breed.
This transgenic fungus caused mosquito populations in their test site to collapse to unsustainable levels within two generations.
“You can think of the fungus as a hypodermic needle we use to deliver a potent insect-specific toxin into the mosquito,” said Leger.
The toxin is an insecticide called Hybrid. It is derived from the venom of the Australian Blue Mountains funnel-web spider and has been approved by the Environmental Protection Agency (EPA) for application directly on crops to control agricultural insect pests.
“Simply applying the transgenic fungus to a sheet that we hung on a wall in our study area caused the mosquito populations to crash within 45 days,” Lovett said.
“And it is as effective at killing insecticide-resistant mosquitoes as non-resistant ones,” he said.
Laboratory tests suggest that the fungus will infect the gamut of malaria-carrying mosquitoes, researchers said.
The abundance of species that transmit malaria has hindered efforts to control the disease, because not all species respond to the same treatment methods, they said.
To modify the fungus Metarhizium pingshaense so that it would produce and deliver Hybrid, the research team used a standard method that employs a bacterium to intentionally transfer DNA into fungi.
The DNA the scientists designed and introduced into the fungi provided the blueprints for making Hybrid along with a control switch that tells the fungus when to make the toxin.
The control switch is a copy of the fungus’ own DNA code. Its normal function is to tell the fungus when to build a defensive shell around itself so that it can hide from an insect’s immune system.
Building that shell is costly for the fungus, so it only makes the effort when it detects the proper surroundings — inside the bloodstream of a mosquito.
By combining the genetic code for that switch with the code for making Hybrid, the scientists were able to ensure that their modified fungus only produces the toxin inside the body of a mosquito.
They tested their modified fungus on other insects in Maryland and Burkina Faso, and found that the fungus was not harmful to beneficial species such as honeybees. (AGENCIES)