By Girish Linganna
In southwest Bolivia, high up in the Andes mountains, there is a massive, shining white salt flat, called the ‘Salar de Uyuni’. It is very flat and looks like a giant mirror. Underneath this salt flat, there is an important alkali metal that is essential for powering today’s technology.
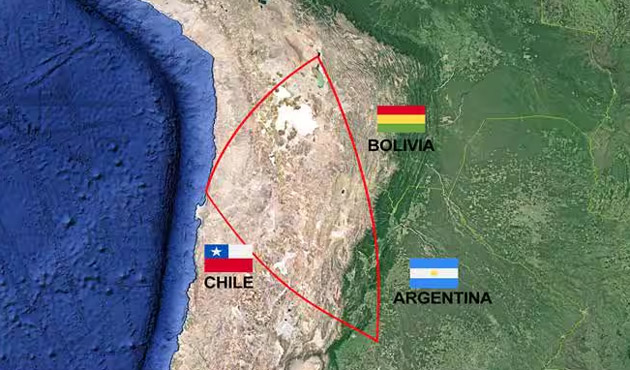
The Salar de Uyuni is located in the Lithium Triangle, an area that includes Argentina, Bolivia and Chile. This region holds the world’s largest lithium reserves—a key component in lithium-ion batteries. These batteries power electronic devices used by billions of people worldwide. In 2022, the worldwide market for lithium-ion batteries was worth $58.21 billion. It is projected to grow at an annual rate of 17.55% from 2023 to 2032, reaching an estimated value of $293.24 billion by 2032.
A key reason for using lithium-ion batteries is to power electric vehicles (EVs), which can help decrease our reliance on fossil fuels. They offer several benefits that make them more popular than other types of batteries. Lithium-ion batteries are rechargeable and found in many items—such as EVs, smartphones, laptops and electric toothbrushes.
Lithium’s small atomic weight and radius are crucial for its role in battery technology, as these properties make the element both light and compact. Consequently, lithium can store a substantial amount of energy in a relatively small space. This characteristic is essential for creating lightweight, high-capacity batteries, making lithium an ideal material for the above applications.
Lithium-ion batteries are crucial for all EVs, including Teslas, and are low in maintenance because they do not require schedule cycling to keep their battery life steady. They also offer high energy density and voltage and can store energy from such renewable sources as solar and wind power. During the battery’s discharge, the anode emits lithium ions to the cathode, creating a stream of electrons between the two sides. When the device is charged, the process reverses: the cathode releases lithium ions that the anode absorbs.
However, producing EVs and, especially, the batteries requires a significant amount of energy and resources. Considering the emissions produced by the transportation sector annually, it is argued that these batteries justify the environmental cost. However, concerns remain about the impact on the planet and on human health.
Although lithium-ion batteries have many benefits, they also pose environmental challenges. While they support renewable energy and generate fewer carbon emissions, there are still some negative aspects to consider. Mining lithium, which is necessary for these batteries, harms the environment. This raises a difficult question: Is it justifiable to damage, and pollute, the environment to extract minerals crucial for supporting the green economy?
Engineers can extract lithium by tapping into underground saltwater, known as brine, through drilling. Once the brine is brought to the surface, it is transferred to evaporation ponds. In these ponds, the water evaporates, leaving behind a concentrated form of lithium that can then be extracted. Nonetheless, reports from the Lithium Triangle paint a grave picture of environmental damage caused by lithium mining.
Every ton of lithium produced necessitates the evaporation of approximately 2 million litres of water. This extravagant water usage leads to significant annual losses and poses a risk to underground freshwater reserves, which can become salinized when they come into contact with the brine.
In the extremely dry regions of South America where widespread mining takes place, water is redirected from local communities to support mining activities. This not only leads to water shortages, but also causes severe contamination issues due to the use of such chemicals as sulphuric acid and sodium hydroxide. Sodium hydroxide and sulphuric acid are used in lithium extraction to separate lithium from other minerals found in the ore or brine.
Community members have reported that mining activities have led to lower water levels in wells, lagoons, groundwater and wetlands, negatively affecting their farming and grazing practices. They have also noticed a higher death rate among flamingos and camelids due to dust pollution from the mines.
Fire Hazards: Lithium batteries are typically safe for use in homes and by individuals, provided they are free from defects. While incidents are rare, there have been cases where lithium ion batteries have ignited, including a cell phone catching fire on a plane, nanotechnology professor at the University of California San Diego Zheng Chen told National Geographic. Additionally, Tesla vehicles and a battery storage facility in Monterey, California, have also experienced fires.
When a battery catches fire, it emits heat, pressure and toxic gases. If there is wind, these gases can spread to nearby residential areas. This is worrisome if there is not an effective strategy in place to mitigate these risks. Chen tells National Geographic that there have been instances of EVs, too, catching fire in garages. Although such events are rare, they still occur. Chen believes it is impossible to completely eliminate all risks, noting that mechanical damage can happen unexpectedly.
To reduce this risk, the Occupational Safety and Health Administration recommends that consumers take lithium-powered devices off the charger once fully charged and store them in dry, cool places. Additionally, they should check the batteries for any signs of damage and keep damaged batteries away from flammable materials, National Geographic states. (IPA)